Research in the Koval Laboratory focuses on understanding how the molecular physiology of lung barrier-forming tissue influences the severity of pulmonary disease. This includes studying the molecular machinery that regulates gap junctions and pannexin large conductance channels (coordinating intercellular signaling), and tight junctions (promoting tissue barriers). Understanding how junctions are pathologically misregulated in lung disease enables us to define pharmacologic targets to control the severity of acute lung injury, sepsis-associated pulmonary edema, and diseases such as cystic fibrosis.
For questions or to learn more about the Koval Lab, please reach out to Michael Koval, PhD, at mhkoval@emory.edu.
Publications
A complete list of our published work can be found at the National Library of Medicine website.
Projects
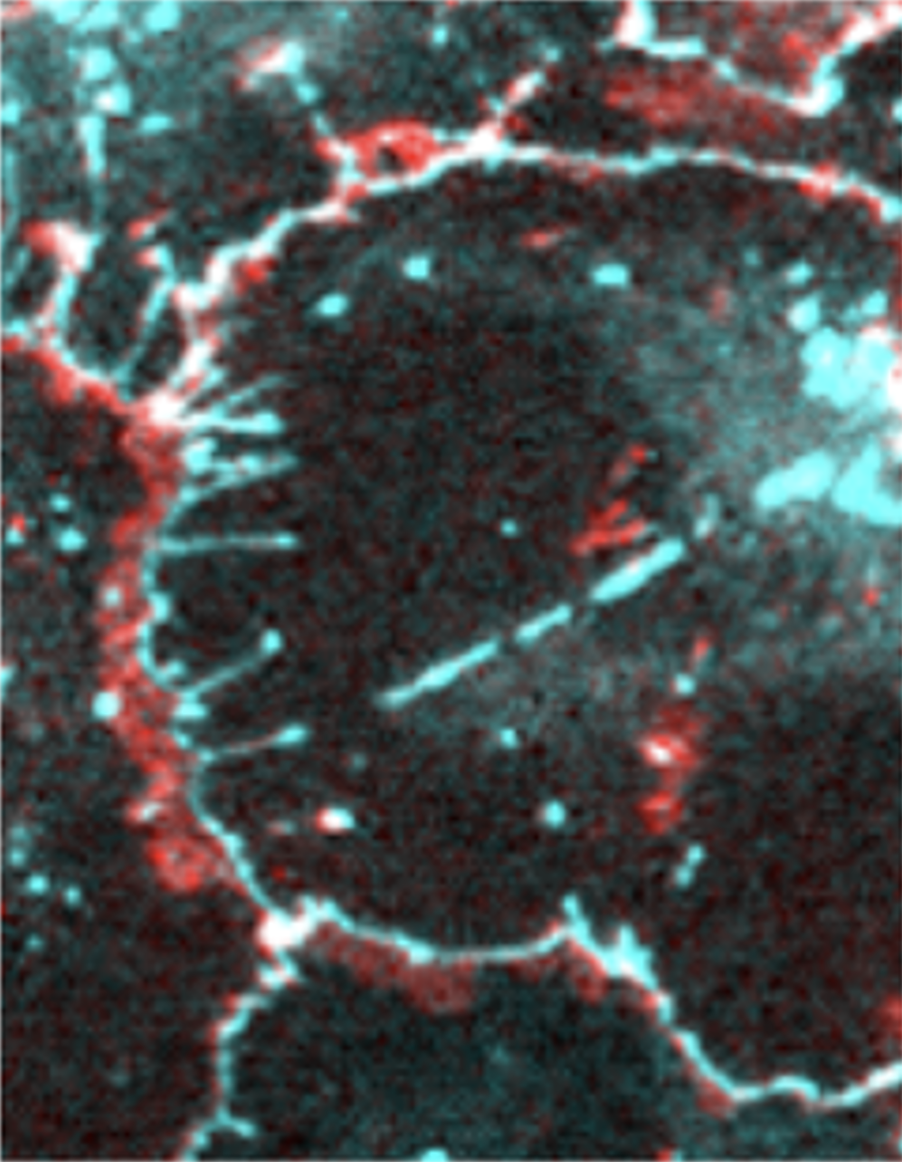
Impact of alcohol on lung epithelial phenotype and barrier function
Alcohol use disorder (AUD) is a significant risk factor for severe acute respiratory distress syndrome because of a deficit in lung barrier function. This is due, in part, to aberrant upregulation of the tight junction protein claudin-5, which puts mechanical stress on cell junctions leading to the formation of tight junction spikes. AUD also causes a phenotypic shift in gene expression in human bronchial epithelial cells, enhancing expression of epidermal genes. Current work focuses on determining mechanisms by which alcohol alters gene expression, defining functions of tight junction spikes as signaling platforms and identifying novel approaches to target tight junction proteins and integrins in order to restore normal barrier function in order to prevent pulmonary edema.
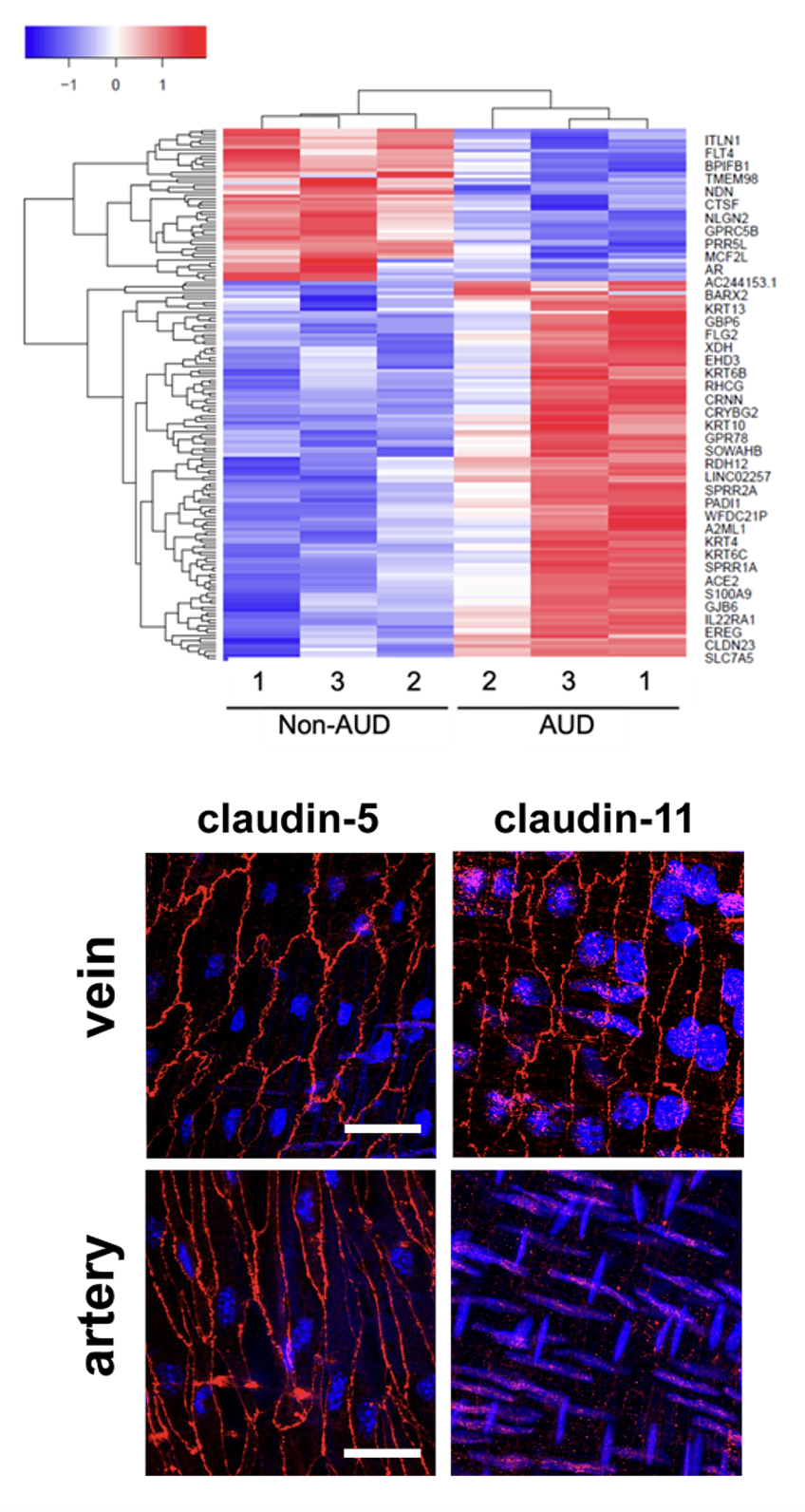
Purinergic Regulation of Veinous Endothelial Permeability
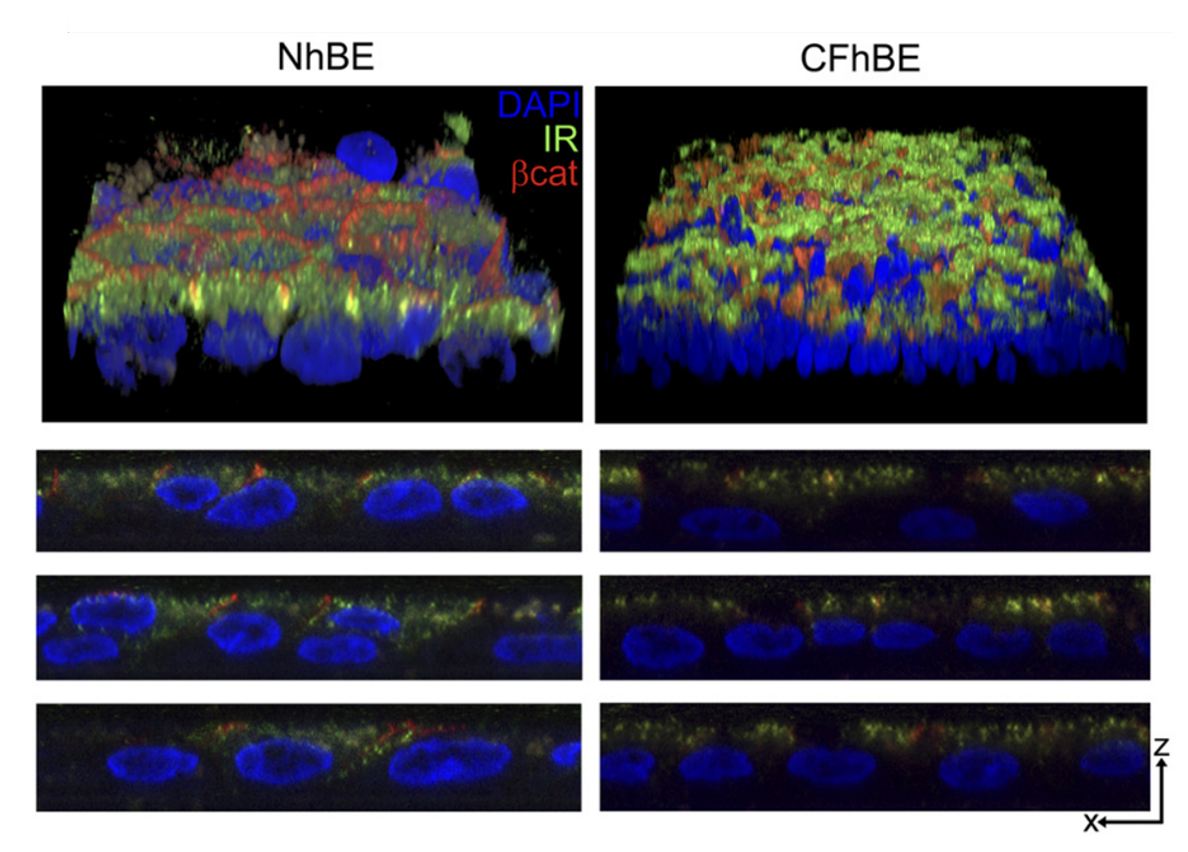
Impact of cystic fibrosis on the airway glucose barrier
Cystic Fibrosis (CF), is one of the most common lethal genetic diseases in the U.S. As CF patients develop CF-related diabetes (CFRD), the most common CF comorbidity, lung function decline is accelerated. Despite the high frequency and substantial clinical significance of CFRD, little is known about how the development of systemic hyperglycemia worsens airway function in CF. In collaboration with Dr. Nael McCarty in the Department of Pediatrics, we have discovered that there fundamental defects in the handling of glucose in the CF airway, including impacts on both the function of tight junction proteins in limiting flux of glucose into the airway and the function of transporters to remove airway glucose from the airway surface liquid, two arms that together create an “airway glucose barrier.” We also discovered that airway epithelial cells express insulin receptors. Insulin-mediated signaling in non-CF cells occurs through the “metabolic” pathway, supportive of tight junction integrity and glucose uptake, whereas, insulin-mediated signaling in CF cells occurs through the “mitogenic” pathway, which is detrimental to barrier integrity. Current work focuses on determining the impact of CFTR mutations on insulin signaling and glucose transport and how this leads to hallmarks of CFRD that impair lung function, including hyperglycemia and airway redox imbalance.


